(16分)工业上可用煤制天然气,生产过程中有多种途径生成CH4。
(1)写出CO2与H2反应生成CH4和H2O的热化学方程式 。
已知: ① CO(g)+H2O(g)
 H2(g)+CO2(g) ΔH=-41kJ·mol-1
H2(g)+CO2(g) ΔH=-41kJ·mol-1 ② C(s)+2H2(g)
 CH4(g) ΔH=-73kJ·mol-1
CH4(g) ΔH=-73kJ·mol-1③ 2CO(g)
 C(s)+CO2(g) ΔH=-171kJ·mol-1
C(s)+CO2(g) ΔH=-171kJ·mol-1(2)另一生成CH4的途径是CO(g)+3H2(g)
 CH4(g)+H2O(g)。其他条件相同时,H2的平衡转化率在不同压强下随温度的变化如图所示。
CH4(g)+H2O(g)。其他条件相同时,H2的平衡转化率在不同压强下随温度的变化如图所示。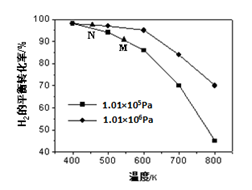
①该反应的△H 0(填“<”、“=”或“>”)。
②实际生产中采用图中M点而不是N点对应的反应条件,运用化学反应速率和平衡知识,同时考虑生产实际,说明选择该反应条件的理由________________________。
③某温度下,将0.1 mol CO和0.3 mol H2充入10L的密闭容器内发生反应CO(g)+3H2(g)
 CH4(g)+H2O(g),平衡时H2的转化率为80%,求此温度下该反应的平衡常数K。(写出计算过程,计算结果保留两位有效数字)
CH4(g)+H2O(g),平衡时H2的转化率为80%,求此温度下该反应的平衡常数K。(写出计算过程,计算结果保留两位有效数字)答案 :
(1)CO2(g)+4H2(g)
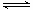 CH4(g)+2H2O(g) ΔH=-162kJ·mol-1 (3分,热化学方程式2分,数据1分)
CH4(g)+2H2O(g) ΔH=-162kJ·mol-1 (3分,热化学方程式2分,数据1分)(2)①< (3分)
②相对于N点而言,采用M点,温度在500-600K之间,温度较高,反应速率较快,氢气的平衡转化率也较高,压强为常压对设备要求不高。 (3分)
③(7分)
CO(g)+3H2(g)
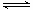 CH4(g)+H2O(g)
CH4(g)+H2O(g)起始时各物质浓度/ mol·L-1: 0.01 0 .03 0 0
平衡时各物质浓度/ mol·L-1 0.002 0.006 0.008 0.008
(以上3分)
K=
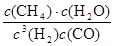 =
=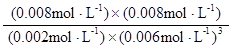 =0.148×106
=0.148×106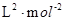
(1分) (1分) (2分,数据和单位各1分)
编辑推荐:
下载Word文档

温馨提示:因考试政策、内容不断变化与调整,长理培训网站提供的以上信息仅供参考,如有异议,请考生以权威部门公布的内容为准! (责任编辑:长理培训)






















点击加载更多评论>>